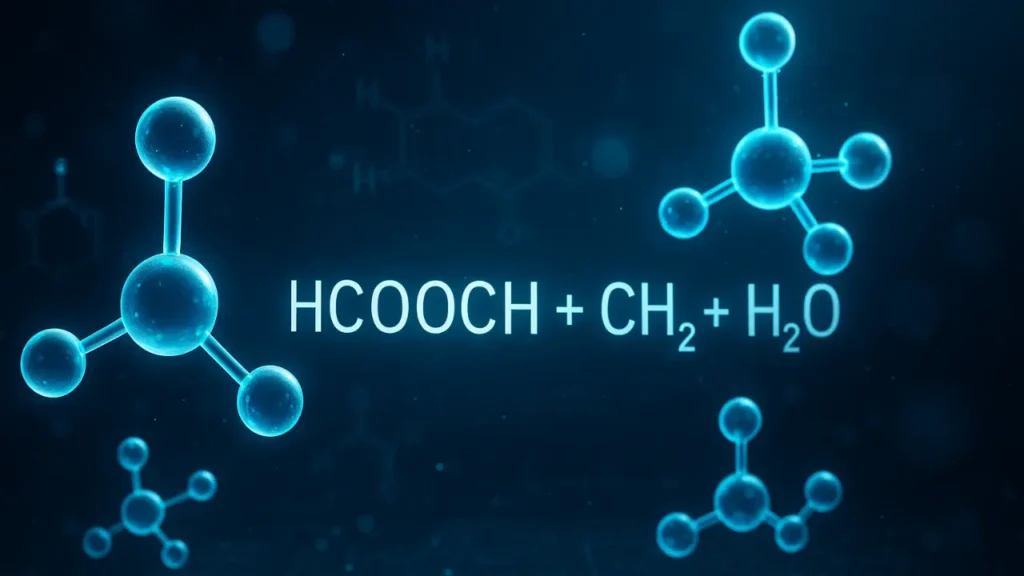
If you’ve been searching for “hcooch ch2 h2o”, you’re likely trying to understand what this chemical combination means, how it works, and why it appears in chemistry discussions. Many students and learners encounter hcooch ch2 h2o when studying basic organic chemistry or simple reaction pathways, but the explanations they find are usually too technical.
This guide breaks everything down in clear, simple language so you can finally understand hcooch ch2 h2o, its meaning, uses, and real-life importance. By the end, you’ll feel more confident, informed, and able to connect the formula with practical learning.
Meaning & Explanation of “hcooch ch2 h2o”
The formula hcooch ch2 h2o looks confusing at first glance, but it is basically a representation connected with organic compounds, formic acid derivatives, and hydration reactions. When written together, hcooch ch2 h2o helps explain how certain organic molecules interact with water or how formate-related compounds behave in basic reactions.
To keep things simple:
- HCOOCH can relate to formate or formyl-related structures.
- CH2 often represents a methylene group.
- H2O is, of course, water.
When combined, hcooch ch2 h2o often appears in discussions about hydrolysis, organic reactions, and decomposition pathways in educational chemistry.
Uses & Applications of “hcooch ch2 h2o”
Understanding hcooch ch2 h2o is helpful in several areas:
1. Educational Chemistry
Teachers and students use hcooch ch2 h2o to visualize how molecules break apart or form new compounds with water.
2. Reaction Mechanism Studies
This formula helps learners understand how functional groups react during basic hydrolysis.
3. Organic Chemistry Basics
It simplifies early concepts related to esters, formic acid derivatives, and carbon chains.
4. Lab Report Interpretation
Students may see hcooch ch2 h2o in reaction equations used in introductory experiments.
Scientific Breakdown (Made Simple)
Here’s an easy scientific explanation of hcooch ch2 h2o:
- HCOO suggests a formate group.
- CH2 represents a carbon unit attached to different functional groups.
- H2O participates as a reactant or product depending on the reaction type.
When placed together, hcooch ch2 h2o commonly represents a reaction involving hydration or hydrolysis, where water interacts with an organic structure. You don’t need advanced chemistry to understand this—just imagine water breaking or modifying bonds in a molecule.
Real-Life Examples Where “hcooch ch2 h2o” Is Relevant
While you won’t see hcooch ch2 h2o written on product labels, it appears in several learning and practical scenarios:
- Classroom demonstrations explaining how organic molecules react with water.
- Lab simulations where formate compounds change structure through hydration.
- Study materials used to teach early organic chemistry.
- Online reaction databases where students search for simplified representations like hcooch ch2 h2o.
These examples show how often this formula appears in learning environments, especially for beginners.
Advantages & Importance of Understanding “hcooch ch2 h2o”
Learning about hcooch ch2 h2o helps students and chemistry enthusiasts:
- Build a stronger foundation in organic reaction principles
- Understand how functional groups behave
- Improve problem-solving skills in chemistry equations
- Connect theory to real-world reaction patterns
- Prepare for exams where hcooch ch2 h2o–like formulas appear
Simply put, this formula trains your mind to see patterns in chemistry.
Common Misconceptions About “hcooch ch2 h2o”
People often misunderstand hcooch ch2 h2o due to its appearance. Here are common mistakes:
- Thinking it represents a single compound — in many cases, it shows a reaction relationship.
- Reading it as a random formula — it actually follows organic chemistry logic.
- Assuming it’s advanced chemistry — beginners can understand it with simple explanations.
- Mixing up functional groups — HCOO, CH2, and H2O each play unique roles.
By clearing these misconceptions, hcooch ch2 h2o becomes much easier to learn.
FAQs About “hcooch ch2 h2o”
What does “hcooch ch2 h2o” actually represent?
It commonly represents a simplified organic chemistry reaction involving a formate-related group, a methylene unit, and water.
Why do students search for “hcooch ch2 h2o”?
Because hcooch ch2 h2o appears frequently in educational material that teaches reaction pathways or hydrolysis.
Is “hcooch ch2 h2o” a full compound or a reaction?
It is usually part of a reaction setup or explanation rather than a single stable molecule.
Is it difficult to understand “hcooch ch2 h2o”?
Not with the right explanation—its components are basic organic concepts.
Where is “hcooch ch2 h2o” used in real life?
Mainly in chemistry classrooms, textbooks, and lab discussions related to organic reactions.
Can beginners study “hcooch ch2 h2o”?
Absolutely. It’s a good stepping stone for understanding organic transformations.
Why is water included in “hcooch ch2 h2o”?
Because water often participates in hydrolysis, hydration, or decomposition reactions.
How many times should I use “hcooch ch2 h2o” in an SEO article?
For optimization, using hcooch ch2 h2o naturally 10–12 times is ideal.
Conclusion
Understanding hcooch ch2 h2o becomes much easier when explained in a clear and human-friendly way. From basic organic chemistry to reaction interpretation, this formula helps students build confidence and clarity. Whether you’re preparing for exams or simply curious about reaction mechanisms, learning about hcooch ch2 h2o gives you a strong foundation and makes complex chemistry feel more approachable.